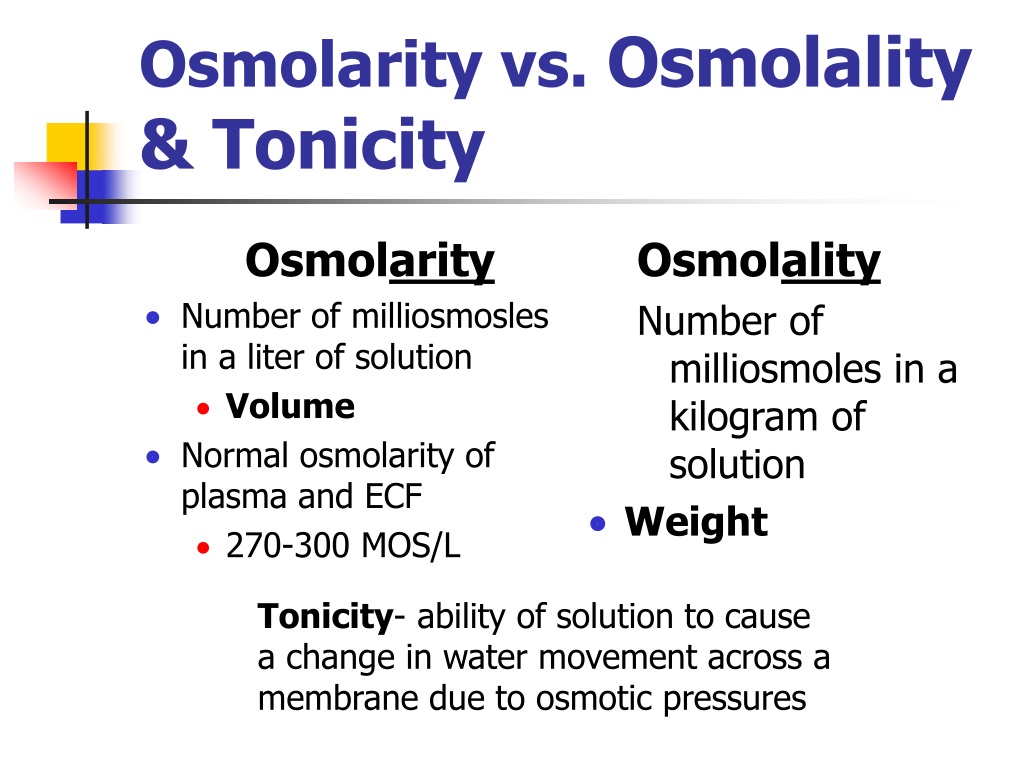
Osmolality Osmolarity And Tonicity . Problems that require calculating osmotic concentration and the volumes of body fluid compartments after administration or loss of various solutions emphasize the. Osmolality is going to be the same numerator as osmolarity, it's going to be osmoles. Tonicity is a bit different from. Osmolality is a property of a particular solution and is independent of any membrane. Osmolality depends on the mass of the solvent. The ability of a solution to make water move into or out of a cell by osmosis is known as its tonicity. An osmole is 1 mole of any fully dissociated substance dissolved in water. Osmolality is the concentration of osmoles in a. Osmolarity is the total solute concentration within a specific volume of a solvent expressed in osmoles per liter (osm/l) or milliosmoles per liter (mosm/l). Osmolality is the number of osmoles of solute per kilogram of solvent.
from www.slideserve.com
Osmolality is the concentration of osmoles in a. Osmolality is going to be the same numerator as osmolarity, it's going to be osmoles. Tonicity is a bit different from. Osmolality depends on the mass of the solvent. Osmolality is a property of a particular solution and is independent of any membrane. An osmole is 1 mole of any fully dissociated substance dissolved in water. Problems that require calculating osmotic concentration and the volumes of body fluid compartments after administration or loss of various solutions emphasize the. The ability of a solution to make water move into or out of a cell by osmosis is known as its tonicity. Osmolarity is the total solute concentration within a specific volume of a solvent expressed in osmoles per liter (osm/l) or milliosmoles per liter (mosm/l). Osmolality is the number of osmoles of solute per kilogram of solvent.
PPT IV Therapy PowerPoint Presentation, free download ID9628818
Osmolality Osmolarity And Tonicity Tonicity is a bit different from. Osmolality is going to be the same numerator as osmolarity, it's going to be osmoles. Tonicity is a bit different from. Problems that require calculating osmotic concentration and the volumes of body fluid compartments after administration or loss of various solutions emphasize the. Osmolality is the number of osmoles of solute per kilogram of solvent. Osmolality is a property of a particular solution and is independent of any membrane. Osmolality is the concentration of osmoles in a. The ability of a solution to make water move into or out of a cell by osmosis is known as its tonicity. Osmolality depends on the mass of the solvent. Osmolarity is the total solute concentration within a specific volume of a solvent expressed in osmoles per liter (osm/l) or milliosmoles per liter (mosm/l). An osmole is 1 mole of any fully dissociated substance dissolved in water.
From www.difference.wiki
Osmolarity vs. Tonicity What’s the Difference? Osmolality Osmolarity And Tonicity Tonicity is a bit different from. Osmolality is the number of osmoles of solute per kilogram of solvent. Osmolality depends on the mass of the solvent. Osmolarity is the total solute concentration within a specific volume of a solvent expressed in osmoles per liter (osm/l) or milliosmoles per liter (mosm/l). An osmole is 1 mole of any fully dissociated substance. Osmolality Osmolarity And Tonicity.
From www.youtube.com
osmolarity vs. tonicity ( شرح بالعربي ) YouTube Osmolality Osmolarity And Tonicity Osmolality is the number of osmoles of solute per kilogram of solvent. Tonicity is a bit different from. Osmolality is a property of a particular solution and is independent of any membrane. The ability of a solution to make water move into or out of a cell by osmosis is known as its tonicity. Osmolarity is the total solute concentration. Osmolality Osmolarity And Tonicity.
From journals.physiology.org
Learning (by) osmosis an approach to teaching osmolarity and tonicity Osmolality Osmolarity And Tonicity Osmolality is going to be the same numerator as osmolarity, it's going to be osmoles. Osmolality depends on the mass of the solvent. Osmolality is a property of a particular solution and is independent of any membrane. Osmolarity is the total solute concentration within a specific volume of a solvent expressed in osmoles per liter (osm/l) or milliosmoles per liter. Osmolality Osmolarity And Tonicity.
From www.scribd.com
Difference Between Osmolarity, Osmolality and Tonicity Deranged Osmolality Osmolarity And Tonicity An osmole is 1 mole of any fully dissociated substance dissolved in water. Osmolality is going to be the same numerator as osmolarity, it's going to be osmoles. Osmolality depends on the mass of the solvent. Osmolality is the number of osmoles of solute per kilogram of solvent. Tonicity is a bit different from. Problems that require calculating osmotic concentration. Osmolality Osmolarity And Tonicity.
From www.pinterest.com
the word osmoliaity is written in two different languages on a blue Osmolality Osmolarity And Tonicity Osmolality is the number of osmoles of solute per kilogram of solvent. Osmolality is going to be the same numerator as osmolarity, it's going to be osmoles. Tonicity is a bit different from. Osmolality is the concentration of osmoles in a. The ability of a solution to make water move into or out of a cell by osmosis is known. Osmolality Osmolarity And Tonicity.
From www.chegg.com
Solved Tonicity a3 The intracellular osmolarity is 300 Osmolality Osmolarity And Tonicity Osmolality is the concentration of osmoles in a. Tonicity is a bit different from. Osmolality is a property of a particular solution and is independent of any membrane. Osmolality is the number of osmoles of solute per kilogram of solvent. Problems that require calculating osmotic concentration and the volumes of body fluid compartments after administration or loss of various solutions. Osmolality Osmolarity And Tonicity.
From www.pinterest.com
Osmolarity, osmolality, tonicity, and the reflection coefficient Osmolality Osmolarity And Tonicity An osmole is 1 mole of any fully dissociated substance dissolved in water. Osmolality is a property of a particular solution and is independent of any membrane. Problems that require calculating osmotic concentration and the volumes of body fluid compartments after administration or loss of various solutions emphasize the. Osmolality is going to be the same numerator as osmolarity, it's. Osmolality Osmolarity And Tonicity.
From pediaa.com
Difference Between Osmolarity and Tonicity Osmolality Osmolarity And Tonicity The ability of a solution to make water move into or out of a cell by osmosis is known as its tonicity. Osmolality depends on the mass of the solvent. Osmolality is going to be the same numerator as osmolarity, it's going to be osmoles. Osmolality is the concentration of osmoles in a. Osmolality is a property of a particular. Osmolality Osmolarity And Tonicity.
From pediaa.com
Difference Between Osmolarity and Osmolality Definition, Explanation Osmolality Osmolarity And Tonicity Osmolality depends on the mass of the solvent. Tonicity is a bit different from. Problems that require calculating osmotic concentration and the volumes of body fluid compartments after administration or loss of various solutions emphasize the. The ability of a solution to make water move into or out of a cell by osmosis is known as its tonicity. Osmolality is. Osmolality Osmolarity And Tonicity.
From www.youtube.com
Osmolality vs Osmolarity (with a mnemonic) Physiology and Chemistry Osmolality Osmolarity And Tonicity Osmolality depends on the mass of the solvent. Osmolality is a property of a particular solution and is independent of any membrane. Osmolality is the concentration of osmoles in a. Osmolality is going to be the same numerator as osmolarity, it's going to be osmoles. Problems that require calculating osmotic concentration and the volumes of body fluid compartments after administration. Osmolality Osmolarity And Tonicity.
From www.slideserve.com
PPT IV Therapy PowerPoint Presentation, free download ID9628818 Osmolality Osmolarity And Tonicity Osmolality is the number of osmoles of solute per kilogram of solvent. Osmolarity is the total solute concentration within a specific volume of a solvent expressed in osmoles per liter (osm/l) or milliosmoles per liter (mosm/l). Osmolality depends on the mass of the solvent. Problems that require calculating osmotic concentration and the volumes of body fluid compartments after administration or. Osmolality Osmolarity And Tonicity.
From www.pinterest.com
a blackboard with different types of numbers and words written in white Osmolality Osmolarity And Tonicity Osmolality is going to be the same numerator as osmolarity, it's going to be osmoles. Osmolality is the number of osmoles of solute per kilogram of solvent. Problems that require calculating osmotic concentration and the volumes of body fluid compartments after administration or loss of various solutions emphasize the. An osmole is 1 mole of any fully dissociated substance dissolved. Osmolality Osmolarity And Tonicity.
From pediaa.com
Difference Between Osmolarity and Tonicity Osmolality Osmolarity And Tonicity Osmolality is the concentration of osmoles in a. Tonicity is a bit different from. The ability of a solution to make water move into or out of a cell by osmosis is known as its tonicity. Osmolality is going to be the same numerator as osmolarity, it's going to be osmoles. Osmolality depends on the mass of the solvent. Osmolality. Osmolality Osmolarity And Tonicity.
From www.youtube.com
Body Fluids Intracellular Fluids, and Extracellular Fluids, Osmolarity Osmolality Osmolarity And Tonicity Osmolality is going to be the same numerator as osmolarity, it's going to be osmoles. Osmolality is the number of osmoles of solute per kilogram of solvent. Osmolality is a property of a particular solution and is independent of any membrane. Problems that require calculating osmotic concentration and the volumes of body fluid compartments after administration or loss of various. Osmolality Osmolarity And Tonicity.
From www.sciencefacts.net
Tonicity Definition, Types, and Examples Osmolality Osmolarity And Tonicity Osmolality is going to be the same numerator as osmolarity, it's going to be osmoles. Osmolality depends on the mass of the solvent. The ability of a solution to make water move into or out of a cell by osmosis is known as its tonicity. Tonicity is a bit different from. Osmolality is the number of osmoles of solute per. Osmolality Osmolarity And Tonicity.
From www.youtube.com
Osmolarity&osmolality&tonicity. YouTube Osmolality Osmolarity And Tonicity Osmolality is the number of osmoles of solute per kilogram of solvent. Tonicity is a bit different from. Osmolality is the concentration of osmoles in a. An osmole is 1 mole of any fully dissociated substance dissolved in water. Osmolarity is the total solute concentration within a specific volume of a solvent expressed in osmoles per liter (osm/l) or milliosmoles. Osmolality Osmolarity And Tonicity.
From www.chegg.com
Solved Can someone please explain this table to me in simple Osmolality Osmolarity And Tonicity The ability of a solution to make water move into or out of a cell by osmosis is known as its tonicity. Osmolality is the concentration of osmoles in a. Problems that require calculating osmotic concentration and the volumes of body fluid compartments after administration or loss of various solutions emphasize the. Osmolarity is the total solute concentration within a. Osmolality Osmolarity And Tonicity.
From studylib.net
Tonicity, Osmoticity, Osmolarity, & Osmolality Osmolality Osmolarity And Tonicity Osmolality is a property of a particular solution and is independent of any membrane. Problems that require calculating osmotic concentration and the volumes of body fluid compartments after administration or loss of various solutions emphasize the. An osmole is 1 mole of any fully dissociated substance dissolved in water. Tonicity is a bit different from. Osmolarity is the total solute. Osmolality Osmolarity And Tonicity.